xenopus laevis pipette|Observations and experiments in the living frog embryo : fabrication This protocol is optimized for rapid in vivo electroporation of Xenopus laevis . pipette with B1–2 mm tip diameter is necessary to electroporate single cells. In order to improve the curing performance, design optimization of autoclave molds has to be performed. In this paper, a finite volume method based numerical model is established to simulate.
{plog:ftitle_list}
Overall, the present study shows that, in the case of mask shortage, decontamination via autoclaving or steaming allows the re-use of masks up to two times for protection against .Learn the basics of autoclaving before you begin. Know what materials you can and cannot autoclave (this is not covered in a lot of detail here). Autoclaving is typically meant to sterilize .
Pipette quality is critical for postinjection oocyte viability. A good quality pipette .Xenopus laevis is a better choice for the teaching laboratory as it is hardier and less expensive. . An intra-oocyte injection method for obtaining the electrophysiological response of follicle-enclosed Xenopus laevis oocytes to an increase in intracellular volume (i.e. stretch) without changing the extracellular medium is described. The response comprised a ‘stretch-activated’ (SA) current which was evoked by injection of an isotonic 14–70 nl droplet and had a transient, .
The strengths of Xenopus laevis and Xenopus tropicalis as experimental model systems include the ability to produce a large number of eggs year-round using injection of gonadotropic hormones to induce ovulation and the entirely external synchronous, stereotypical development following fertilization [1,2,3,4].The fertilization protocols are straightforward and .
This protocol is optimized for rapid in vivo electroporation of Xenopus laevis . pipette with B1–2 mm tip diameter is necessary to electroporate single cells. Here, we describe methods for in vivo-targeted electroporation of single tectal neurons within the albino Xenopus laevis tadpole. Focal electroporation is achieved using a pipette electrode filled with a solution of the delivery molecules and with a tip diameter much smaller than the width of the target cell. The small tip allows for . The Xenopus laevis embryo is a model of choice to study NC development. Large numbers of embryos are easy to obtain, and external fertilization gives access to the very first steps of development. . Pipette 500 ml of 10 mg/ml culture grade fibronectin diluted in 1x Phosphate Buffered Saline (PBS) into a standard plastic Petri dish (Ø 40 x 11 .
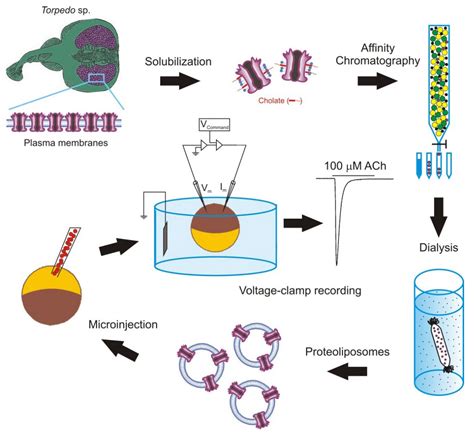
Cell-free extracts derived from the eggs of Xenopus laevis remain a robust and versatile system to decipher the biochemical steps underlying this essential . immediately above the debris is the oily layer above which is the mitochondrial layer). Use a cut pipette tip (P1000 or P200) to recover the desired fractions (Figure 1E). Supplement .Pipette Solution. Oocytes Stripping Solution. Xenopus oocytes (Newman et al. 2017) . This protocol is based on the study of voltage and Ca 2+-activated BK channels exogenously expressed in Xenopus laevis oocytes using patch-clamp techniques (Zhang et al. 2017). By excising inside-out patches, the cytosolic side of the channel can be exposed .The South African frog Xenopus laevis has been used for a number of years as a laboratory animal and for its oocytes (see Note 1.see also refs. 1–3). Xenopus oocytes provide several advantages in addition to their ability to efficiently translate heterologous cRNA into the respective protein, and to dock and insert functional membrane proteins of mammalian (or other) origin .For more than 30 years we have used cell-free extracts prepared from the eggs of the South African clawed frog, Xenopus laevis, to study the control of cell cycle progression and DNA replication.Originally conceived to study chromatin remodeling [], this embryonic model system is used to conduct research in fields as diverse as DNA replication, apoptosis, nucleocytoplasmic .
First, the glass pipette records cells in a limited area in the tissue because the pipette and the tissue make an electric shield, excluding signals outside of the pipette. Excluding signals outside of the pipette contact area makes spike sorting easy. In addition, neurons within the cell-dense layers of the Xenopus tectum are closely packed .
Observations and experiments in the living frog embryo
Note that injection pipette is out of focus. . The heterologous expression system in Xenopus laevis oocytes was previously confirmed to be sensitive enough for identification of mRNA and its . Xenopus laevis egg extract is a well-established system to recapitulate and study complex cellular processes in an in vitro context. . Using a plastic Pasteur pipet with the tip cut off, farm out white puffy activated eggs or lysed eggs. Only retain eggs with distinct and equal dark animal poles and white vegetal poles.
izod hardness test
handling both Xenopus laevis and Xenopus tropicalis,wehaveidentified critical points at which methodsthatworkforX. laevis do not necessarily work well for X. tropicalis (e.g., Hirsch et al. . pipette (e.g., Fisher 13-711-7M) to remove eggs from the frog epidermis while avoiding any injury toXenopus laevis. Oocytes: Reconstitution of Abscisic Acid Activation of SLAC1 Anion Channel via PYL9 ABA Receptor . Cun Wang. 1, 2, 3, *, Jingbo Zhang. 1. . Fill pipette tip with mineral oil to about one-third to two-thirds. 5. Mount pipette to the microdispenser. 6. Deposit cRNA sample onto Parafilm, to a total volume of cRNA > 1 μl.Abstract. Nearly a century ago, studies by Lancelot Hogben and others demonstrated that ovulation in female Xenopus laevis can be induced via injection of mammalian gonadotropins into the dorsal lymph sac, allowing for egg production throughout the year independent of the normal reproductive cycles. Hormonally induced females are capable of producing thousands of eggs .
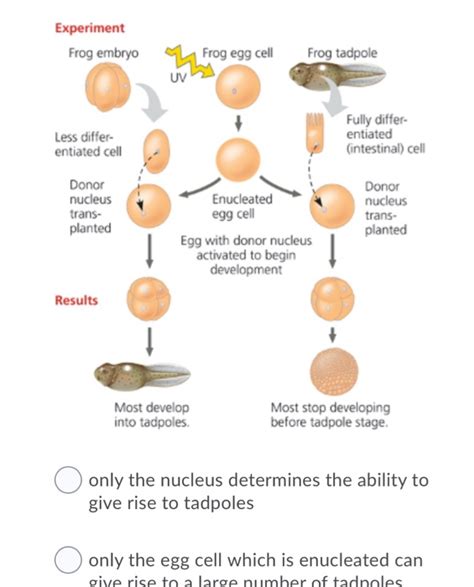
Construction of the Xenopus cell landscape using Microwell-seq. Xenopus laevis is a classical model of vertebrate embryonic development 1, regeneration potential 8, and neuronal regulation 21 .ABSTRACT. An analysis has been made of factors which can cause abnormal development of embryos derived from transplanted nuclei in Xenopus laevis. A distinction is made between innate and technical factors, and between those which affect donor nuclei and recipient eggs.The innate developmental potentialities of endoderm donor nuclei do not appear to be limited, at least up . 3.1 Tadpole Rearing. Xenopus laevis embryos and tadpoles are reared at 20 °C in chlorine-free water (photoperiod = 12 h/12 h light/dark), in groups of 70 individuals per 15 L tank. All procedures are performed in accordance with European Union Directive 2010/63/EU. 3.2 Treatment with Thyroid Hormone T 3. 1. Make a 10 −4 M intermediate solution of T 3 fresh .
Microinjection of Xenopus Oocytes
Xenopus laevis tadpoles of either sex, staged 50–55 (staged after ). . 3.2 Pipette Fabrication for Sparse Cell Labeling in the Brain 1. Fabricate electroporation micropipettes from borosilicate glass capillaries using a horizontal micropipette puller. 2. Modify the protocol to . Once dissociation was complete, individual cells were transferred to a PCR tube using a 10 μL pipette tip under a microscope. Cells were flash frozen in liquid nitrogen and stored at −80 °C until cell lysis and protein extraction. . The Xenopus laevis database was downloaded from UniProt in March 2019 (Proteome ID: UP000186698) for single .
Xenopus laevis, a classic model system for the study of early neural development 19,27,29,31-32,40-42, . The pipette containing the tissue should be fully submersed in plenty of fluid and the cells or tissue expelled very slowly. After the cells have been plated, all fluid transfers including, Fluo4-AM addition, and Cell Culture Medium, or .In this protocol, messenger RNA of BK channels is injected into Xenopus laevis oocytes (stage V-VI) followed by 2-5 days of incubation at 18°C10-13. . First, remove the electrode wire from the pipette holder and submerge the tip half of the electrode wire in a vial containing fresh bleach for at least 15 minutes. This deposits a layer of .
(B) MscS channel activity in oocytes, recorded in 96 mM NaCl plus 2 mM KCl (ND96). (C) Opening and closing of single MscS channels in response to stretch in an excised Xenopus oocyte patch. Pipette, BN 5. (D) Single-channel conductance measured in 98 mM TEA-Cl. Pipette, BN 7. Pipette potential was +40 mV in A and B, +30 mV in C and D.
Overlay with a 1″ square of plastic cut from a pipette tip box or a 1″ square weigh boat. Remove after the agarose hardens. Seal with film and store at 4°C. . Li M, Rohrer B (2006) Gene silencing in Xenopus laevis by DNA vector-based RNA interference and transgenesis. Cell Res 16:99–105. Article PubMed CAS Google Scholar . We demonstrate this protocol on Xenopus laevis embryos treated with a retinoic acid receptor inhibitor that induces abnormal orofacial development and a median cleft palate 2,3. . Modify a pipette tool. Place the tip of a glass Pasteur pipette into a flame from a Bunsen burner. Rotate the pipette tip until it melts and forms a rounded sealed end.
This protocol describes a method to visualise ligands distributed across a field of cells. The ease of expressing exogenous proteins, together with the large size of their cells in early embryos, make Xenopus laevis a useful model for visualising GFP-tagged ligands. Synthetic mRNAs are efficiently translated after injection into early stage Xenopus embryos, and injections can be . Although the genus contains more than a dozen species, of varying ploidy, the two species most commonly used in biomedical research are Xenopus laevis (the South African clawed frog) and Xenopus tropicalis (the West African clawed frog) [1]. These two species of African aquatic frogs are members of the family of tongue-less frogs, known as Pipidae.
jade hardness test
jominy hardness test
Our Vision is to design, develop and manufacture indigenous products conforming to the international quality standards & providing the same to our customers at a much more .
xenopus laevis pipette|Observations and experiments in the living frog embryo